Watch This Video Before Consuming Digene Gel
Abbott India recalls Digene Gel due to taste and odor issues reported by DCGI.
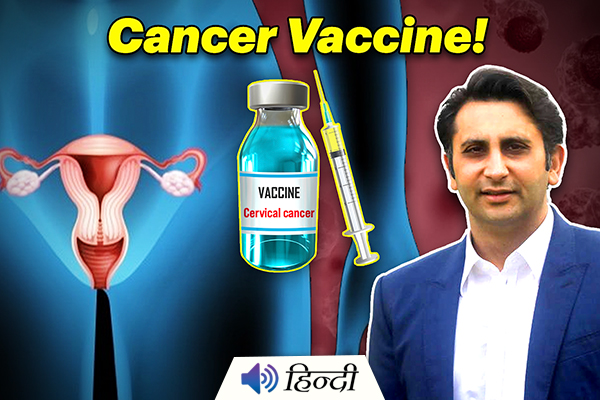
India Gets its First Home-Made Vaccine for Cervical Cancer
Serum Institute of India CEO Adar Poonawalla on Tuesday 12th, July said that the company plans to launch its developed Indian vaccine to treat cervical cancer in women by the end of this year.
PM Modi Announces Booster Doses & Vaccines For Children
India will start vaccinating children between the ages of 15 to 18 from January 3 next year, Prime Minister Narendra Modi announced in a sudden address to the nation.
DCGI : Gautam Gambhir Stored COVID Drugs Illegally
The DCGI has told the Delhi High Court that Gautam Gambhir illegally purchased and distributed medicine and oxygen.
COVAXIN Vaccine Trials Begin on Children in India
Drugs Controller General of India (DCGI) granted COVAXIN approval to carry out clinical trials on children on May 11.
DCGI Approves DRDO's Anti-Coronavirus Medicine
Drugs Controller General of India (DCGI) has approved the emergency use of an anti-coronavirus drug developed by a laboratory of the DRDO.
‘Feluda’ Tests Approved for Testing COVID-19
Drugs Controller General of India (DCGI) of India has approved commercial launch of Feluda a low cost COVID-19 test.
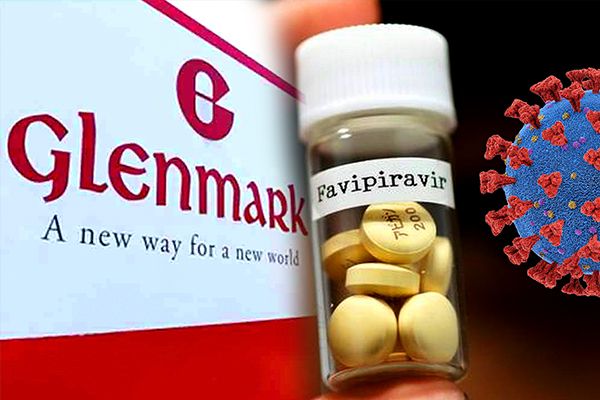
Glenmark Launches Favipiravir Medicine For COVID-19
After receiving permission from Drugs Controller General of India (DCGI), Glenmark Pharmaceuticals launched the antiviral drug Favipiravir for the treatment of mild to moderate COVID-19 cases.